Case Studies
SOLUTIONS DRIVEN
Case Study – Buffer Delivery System
SUMMARY
A young life-science company required a buffer solution storage and delivery system for their novel diagnostics platform.
The project was split into two phases – firstly to provide an on-board buffer package with the required shelf life, and secondly, to provide a reliable, repeatable buffer release puncture method.
Both phases were successfully delivered, allowing the company to progress to the next stage of their product development, satisfy investors, and enable further funding.
MAIN CHALLENGES
- Providing 12 months shelf life of a pre-determined volume of buffer, in a flexible blister pouch with easily punctured lid.
- Working within a fixed space within an existing device and analysis instrument – into which any additional puncture mechanism would need to be accommodated.
- Meeting a minimum and maximum puncture force requirement already defined by the analysis instrument.
- Ensuring products would reliably puncture at point of use, and prematurely during storage and handling.
- To provide a scalable solution, which indicative production costs for future large-scale manufacture.

20 cards ready to test
Blister Pouch Compression Test

Bucket 3D prints in bottomless card

Bucket components

Wash Pouch
PHASE 1 APPROACH
- A materials comparison exercise to identify a range of suitable substrates, to go forward to part development and testing based on security of supply, formability, seal-ability, and moisture barrier.
- Physical testing of materials, partnered with projected calculation of annual moisture loss, to enable a data-driven choice of the best material combination.
- Functional prototype manufacture, enabling stability testing to demonstrate that initial shelf life was in line with theoretical values.
- Longer term shelf-life testing carried out whilst the next phase of the project was undertaken.
PHASE 2 APPROACH
- Concept generation of various pierce and burst mechanisms, leveraging experience within FlexMedical team, and engaging industry expertise.
- Use of 3-D print and Vacuum Cast prototyping to allow quick turnaround of functional tests on concepts, which could be ruled in or out, or modified based on the data.
- Extensive testing regime to verify design to allow investment in Prototype Injection Mould tooling.
- In-house assembly of mouldings with puncture needle and sealing mechanism to provide initial batch of pre-production modules for testing.
RESULTS
- FlexMedical solutions were able to deliver both phases of the project successfully.
- The empirically measured shelf life conformed very closely to the theoretical calculations initially made, and thus within the value specified by the customer.
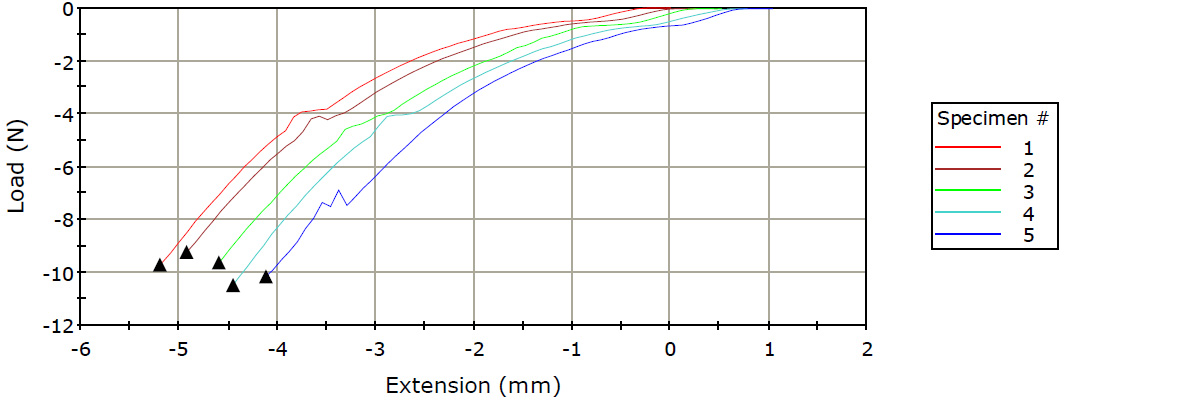
- Modules were shown to puncture within the specified window of applied force.
- Buffer fluid was delivered to the rest of the device successfully, when required.
- The customer was able to meet investor milestones.
- The customer could proceed to the next stage of product development.