Wearable Sensor Development (Step 1 of 5)
When pulling together our development project, we decided that we should use lactate as our target for measurement. Lactate is a byproduct of anaerobic glycolysis which is associated with a range of clinical disease states but also has applications in sports science. Additionally, it’s recognised as one of a combination of markers linked to sepsis, so is an interesting molecule. From a biochemical point of view, it is measured enzymatically similar to many other clinical chemistry analytes. Therefore, it demonstrates proof of principle on lactate, using this as a stepping stone to rapid development of other enzymatic continuous biosensors. Lactate is present in blood, interstitial fluid, and sweat, and is therefore readily available – perfect for this project.

For the purposes of this blog, we have split the process into 5 steps:
Step 1: Electrochemical Reaction
The initial step is to establish the assay biochemistry using the core building blocks intended to be used in the final device. These core building blocks are typically: working and counter electrode material, reference electrode material, substrate, and biochemicals.
It’s a great way to kick off an assay development project as it verifies the activity and performance of the key raw materials and starts the project with positive momentum. At this stage in the project because there is still a lot of latitude, this first step should inevitably work.
This approach reflects a strong conviction that successful diagnostic device, or for any other kind of biosensor, development needs to start with the elements of the system that are abstract and underpin the core technology (the chemistry). By locking down decisions related to macro components (the test strip, the cartridge, the reader) too early you can unintentionally limit options available in the scientific solution. Get the science to do the heavy technical lifting at the start and thus allowing more flexibility around the design of the tangibles (strip, cartridge, reader) but be prepared to go fast on these elements.
Key materials were chosen that are planned to be used in the final prototype. Reagent design was based on a design structure we were confident would yield a positive outcome. In the first iteration we want to see clear dose response where precision is at a place that allows you to have confidence in the separation between concentration levels. Additionally, you should be able to demonstrate sensitivity and linearity across the intended dynamic ranges (not final performance, but sufficient to give you confidence that through further optimisation the required performance will be met). Two enzyme types were used, both with varied characteristics and benefits, and both were included in the reagent at equivalent final activity per sensor.
At this stage the development is being done on standard FMS-008 electrodes. We use these electrodes as a very stable and consistent reliable platform that allows the team to rapidly bring the biochemistry to life, and whereby we have a known pathway from wet E-chem on our “off the shelf” design to equivalent or better performance in a dry lidded bespoke biosensor format.
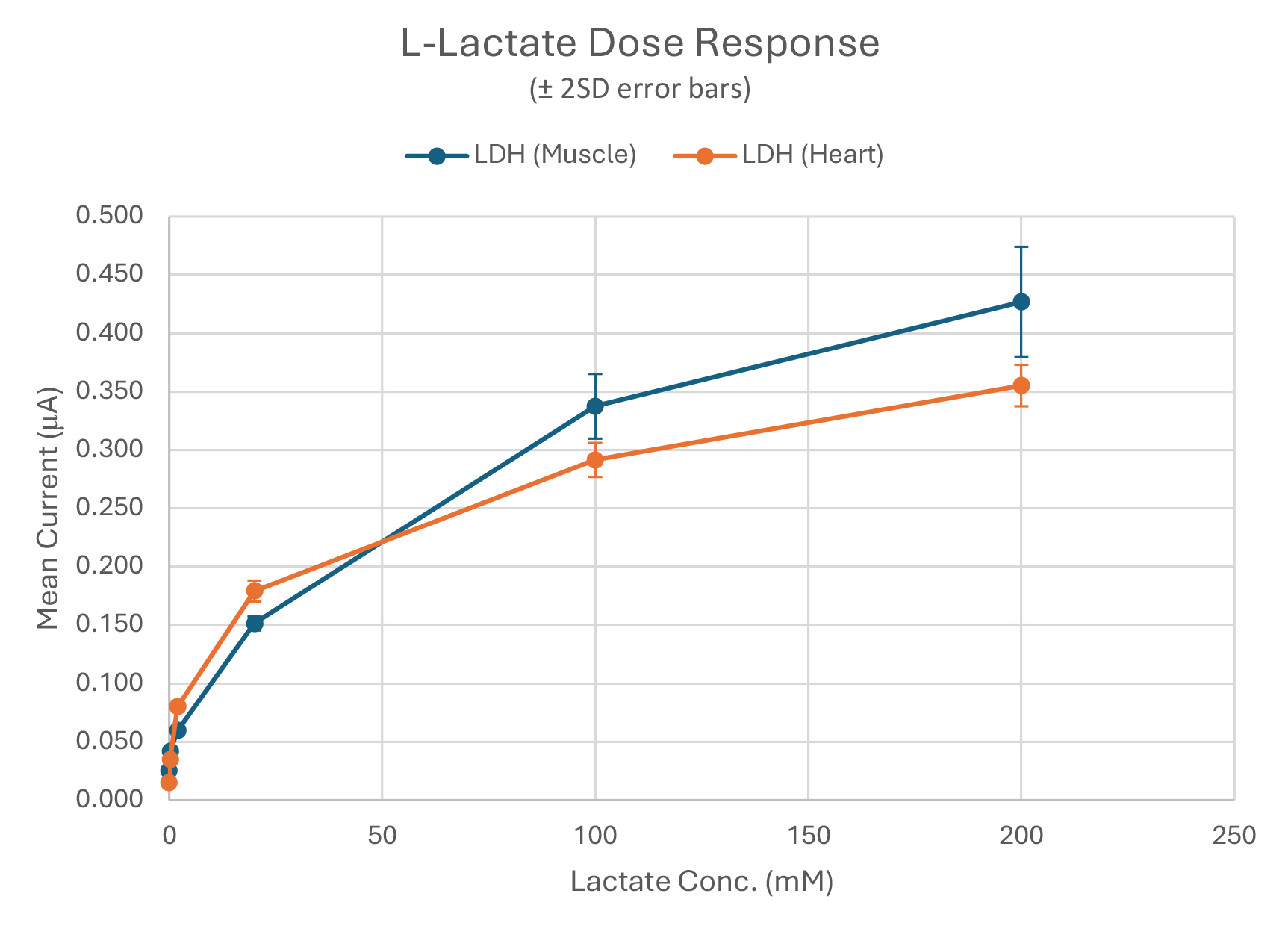
Our sensitivity target was achieved with both enzymes 3.4µM (Heart) and 24.7µM (Muscle); and equivalent mean % CV was achieved in this early data: 5.1% (Heart) and 3.0% (Muscle).
Linearity was observed to be qualitatively better for (Muscle) compared to (Heart).
With the core electrochemical pathway proven and biochemicals tested on the sensor materials, the next major step will be demonstrating dry performance of the enzyme.
Most biochemists can swiftly build a demonstratable wet electrochemical reagent – the real skill is to design the excipient components (and deposition and drying settings) to ensure homogeneous passive resuspension/rehydration and retention of full activity through a long shelf life in ambient storage conditions (cost effectively!)
We’ll see how good a job we’ve done in our next update…….
Donogh FitzGerald
Chief Technology Officer